- API
- Ivabradine hydrochlorid
- Rivaroxaban
- Apixaban
- Dabigatran etexilate
- Safinamide
- Ticlopidine hydrochloride
- Mirtazapine
- Clopidogrel bisulfate
- Duloxetine hydrochloride
- Rebamipide
- Intermediates
- Duloxetine intermediate
- Clopidogrel intermediate
- Mirtazapine intermediates
- Dabigatran etexilate intermediates
- Lurasidone intermediates
- Bepotastine intermediates
- Asenapine Intermediates
- Drotaverine intermediates
- Prasugrel Intermediates
- Ivabradine、Verapamil, Denopamine intermediates
- Moxifloxacin intermediate
- Ticagrelor intermediate <Under Development>
- Canagliflozin intermediate
- Ivabradine intermediate
Address: Zhejiang Provincial Chemical and Medical Raw Material Base Linhai Zone, Duqiao Town, Linhai City, Zhejiang Province, 317016, China
Tel / Fax: 0576-85588211
Tel / Fax: 0576-85588211
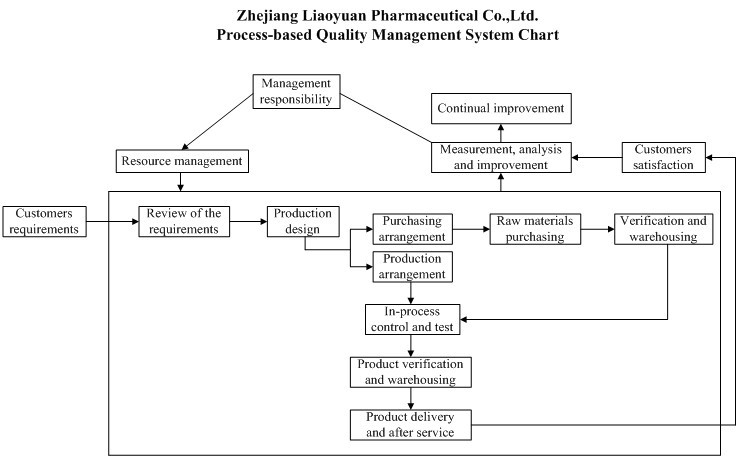
| Liaoyuan has established advanced QC labs; equipped with experienced analysts and advanced imported analytical equipments. The Pharmaceutical production is strictly analyzed and inspected according to C-GMP standard, which effectively supports manufacture, research and development of all products. With strict analysis and management procedure, Liaoyuan pays great attention to the product stability test, validation and transfer of analysis method,and out-of–specification (OOS) investigation, which ensures the accuracy and scientific of analytical data. |
| Quality First” consciousness of the whole staff has been comprehensively improved by means of emphasizing the importance of quality and strengthening employee-skill training. Advanced equipment and scientific management contribute to excellent managing talents and high-quality products. Official audit authority and customer quality auditing institutions at home and abroad are all very satisfied with the perfect management and superior-quality products after each auditing. |